- Showing results for
- Asia Pacific
Taiwan’s solar generation reached 12.9 billion kWh in 2023, providing almost 48% of Taiwan’s total renewable energy generation. The focus of the government on energy security and sustainability aligns with the expansion of solar energy infrastructure. The growing electricity demand is pushing the need for additional solar photovoltaic (PV) installations, particularly in industrial and commercial sectors, which are significant consumers of electricity in Taiwan. Moreover, the energy requirements of the industrial sector are driving the adoption of large-scale solar PV projects. In parallel, Taiwan's battery market is expected to reach USD 0.77 billion by 2025 and grow at a CAGR of 14.3% to USD 1.49 billion by 2030. The government plans to accumulate 590 MW of battery-based energy storage by 2025, with significant contributions from both public and private sectors.
2025-05-20 14:36:13
In recent years, Taiwan has emerged as a significant player in the global medical device industry—an ascent shaped not only by its advanced manufacturing capabilities but also by bold regulatory transformation. At the heart of this transformation is the Medical Devices Act, a landmark piece of legislation that redefined how medical technologies are developed, approved, and marketed within Taiwan. Driven by the need to align with international standards and respond to the growing complexity of modern medical technologies, the Act has introduced a risk-based regulatory framework, streamlined approval processes, and facilitated global market access. These reforms have strengthened Taiwan’s position as a competitive and trusted source of medical devices for global healthcare markets. Taiwan's medical device industry is now undergoing rapid growth, propelled by this regulatory clarity, continued investment in high-tech innovation, and rising global demand for safe, effective, and export-ready medical solutions. For manufacturers and investors looking to access the international medical device market, Taiwan offers a strategically evolving landscape that is both business-friendly and globally connected.
2025-05-20 14:46:38
As augmented reality (AR), virtual reality (VR), and smart devices redefine how we interact with the digital world, one component lies at the heart of this transformation: the optical lens. These precision-engineered components enable everything from immersive simulations to advanced camera features and real-time data overlays. In this rapidly expanding sector, Taiwan has established itself as a strategic global hub for innovation, manufacturing, and partnership. With decades of expertise in optics, a robust high-tech supply chain, and strategic integration with the semiconductor and display industries, Taiwan is not only keeping pace with demand—it’s helping to shape the future of visual technology.
2025-05-20 14:55:08
With its robust semiconductor industry, advanced manufacturing capabilities, and strategic investments, Taiwan contributes significantly to the production and innovation of memory technologies essential for various applications, from consumer electronics to data centers.
2025-05-20 15:01:18
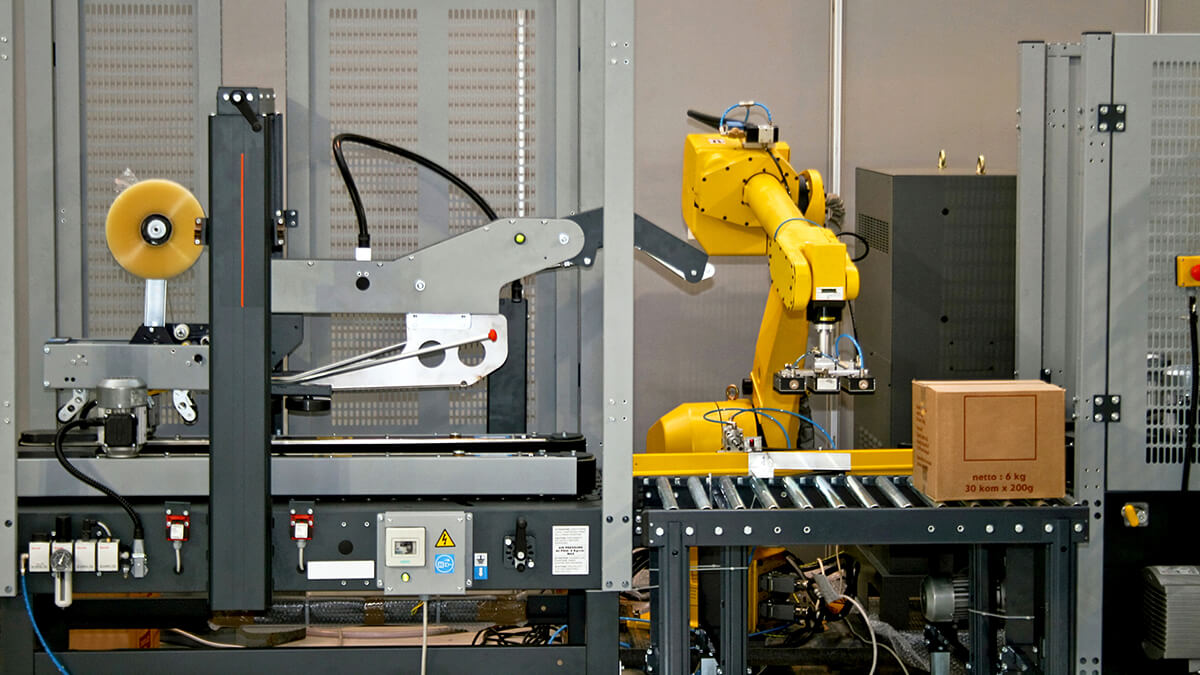
Package Filling Machines integrated with AI Vision Systems offer advanced solutions for efficiently packaging food and powdered substances into precise, small-format packets. These food-grade systems combine mechanical precision with artificial intelligence to ensure quality, regulatory compliance, and high productivity.
2025-05-20 15:08:18
As packaging lines embrace greater automation and data integration, smart labeling has become a vital component of packaging systems. While AI vision systems enhance visual inspection and quality control, smart labeling technologies help manage data, ensure compliance, enhance traceability, and enable real-time customization in the packaging process.
2025-05-20 15:38:39
As the demand for high-performance computing, AI, and data-intensive applications grows, the need for efficient and thermally optimized memory solutions becomes paramount. As system-on-chip (SoC) architectures evolve and Artificial Intelligence (AI) and Machine Learning (ML) workloads surge, the need for efficient memory and reliable heat management is more critical than ever. Recent breakthroughs in flash and DRAM technologies are not only enhancing performance but also addressing critical heat management challenges. Taiwan, a global leader in semiconductor manufacturing, is at the forefront of these innovations.
2025-05-23 11:17:00
The global rollout of 5G technology has been a catalyst for rethinking the materials used in next-generation devices. Unlike previous mobile generations, 5G relies heavily on millimeter wave (mmWave) frequencies, which offer faster data speeds but are more vulnerable to interference. With escalating demands for electromagnetic interference (EMI) shielding and thermal regulation in compact, high-frequency environments, advanced plastics are becoming the backbone of 5G infrastructure. Taiwan manufacturers are engineering high-performance polymers that meet the complex requirements of these modern telecommunications.
2025-05-23 11:25:46
Agree